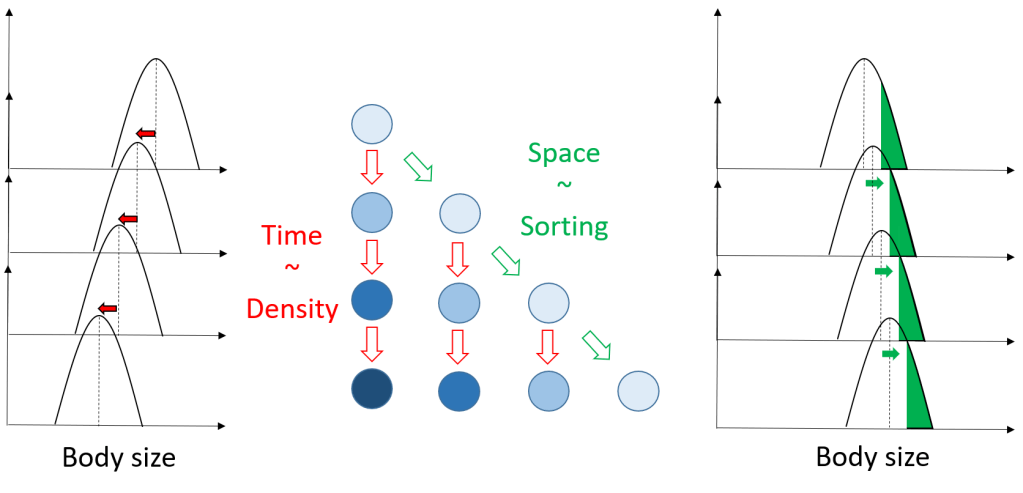
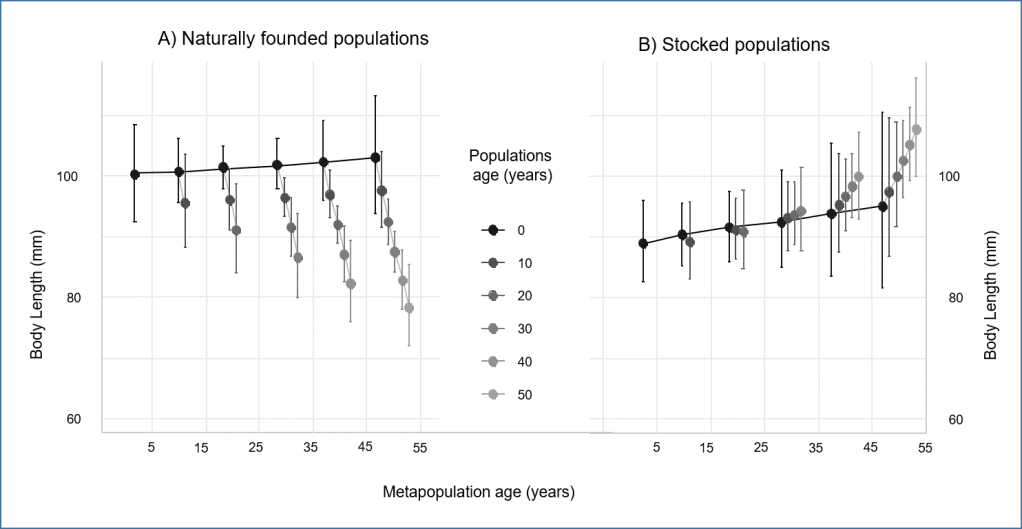
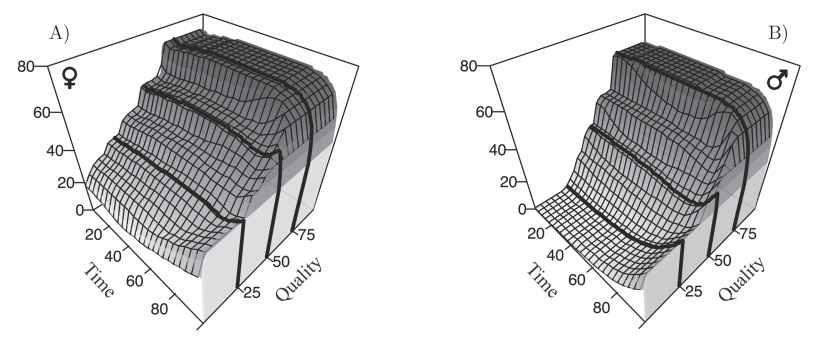
The modern synthetic theory of evolution is often referred as to one of the most successful scientific theories. This is so, because in a handful of principles, mechanisms, it seems to have the power to explain many different things in our environment. Macro-evolution, paleontology, artificial selection, heredity, etc. It ripples in many biological sciences, possibly bringing some « light » to make sense of what surrounds us, as coined by Dobzhansky. To the researcher however, nothing looks very obvious in this theory, or we would not still be trying to test all its premises and predict all its consequences.
This theory mainly deals with the interacting effects of four forces: mutation, genetic drift, gene flow, and selection. The interplay of these forces has yet to be fully explored, understood, assimilated. We’re still far from that. The last one, selection, is the founding principle behind Darwin’s thinking of course. It mainly describes the inequality between individuals in terms of genetic contribution to the next generation. The shape, the strength, the speed of selection are of major interest, because they are expected to be seminal to local adaptation and divergence between gene pools located in different environments. But all these mathematical descriptors of selection hide a simple truth: selection emerges from interaction between individuals within a given environment.

Interaction between individuals is one of the hardest things to predict in science. This is so because individual decisions are made all the time depending on informations: internal and external informations, both possibly changing at a rapid pace. Indeed, other individuals decide too, react to their internal state and their direct environment. And this environment is changing dynamically. In a nutshell, it is mostly about local context.
On the one hand, one can decide to exactly look at this context, and its conditional choices. As an example, Game Theory has been specifically developed to this intent, and therefore provides us with a rational expectation of what individuals should do when facing a decision, with total or partial information. This approach embraced by Maynard-Smith however, as several others, is burdened (but also empowered) by optimality assumptions, that are consubstantial to behavioural ecology: individuals, at evolutionary equilibrium, should choose what is best for them, because if they do not, then it is not an equilibrium: they will reduce their fitness. What is unclear is whether actual evolution will reach such equilibrium: yes selection is here, but remember about mutation, drift, gene flow ? As many hurdles on the path to equilibrium.
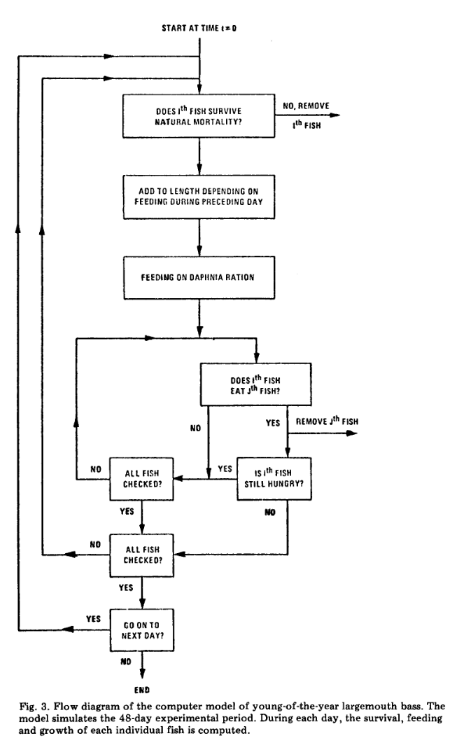
On the other hand however, one can actually decide to let the play unroll, and observe the result. For instance, De Angelis et al., in 1980, could not fathom why from initially similar experimental conditions, very qualitatively different patterns could be obtained, for instance, the emergence or the lack of cannibalism in fish tanks. Turning his attention to the interactions between individuals, he realized that their outcome could be very contrasted, and therefore did not lead to a single equilibrium, but to several, fundamentally, qualitatively, and ecologically different. Such an outcome would likely not be obtained using game theory for instance. This observation was seminal to the development of agent based modelling, where the focus is directed to the algorithmics of interactions between agents, and the resulting and emerging patterns (see the Figure extracted from de Angelis et al., 1980).
Being ecologists, being geneticists, eco-physiologists, behaviouralists or demographers, we realize that we produce knowledge pertaining to natural selection, but we always do so in a specific context. And yet, we are tempted to derive general rules from our results, whereas we usually have a feeble grasp of the interactions between individuals in our experiments. Either because it was not the focus of the experiment, or because we could not produce many different and replicated experimental situations. One way however to explore the field of possible outcomes is to turn to dynamic modelling, involving both the 4 driving forces of evolution, and the individual interactions that give rise to them.
Being ecologists, being geneticists, eco-physiologists, behaviouralists or demographers, some of us have already turned to this solution, and it is proving to be enlightening. In particular, it rapidly reshapes what we thought to be the main drivers of evolution, the speed at which they can operate, and how much selection is context dependent. Some of us also felt the need to give a name to this approach, so to identify scientific studies that integrate the required ingredients to study eco-evolutionary loops in a realistic framework: DemoGenetic Agent Based Models, or DG-ABMs.
I have been quite lengthy already, so if you want to know more about this new generation of eco-evolutionary models, you can either check out the summary figure below, or have a look at our last paper on the matter.

Reference cited:
DeAngelis DL, Cox DK, Coutant CC. 1980. Cannibalism and size dispersal in young-of-the-year largemouth bass: experiment and model. Ecological Modelling. 8:133–48